Neuromodulation
Visit the HNP website to browse through the available equipment, skills and staff at your disposal; enquire about new projects or learn more about studies and publications that have benefited from the facility.
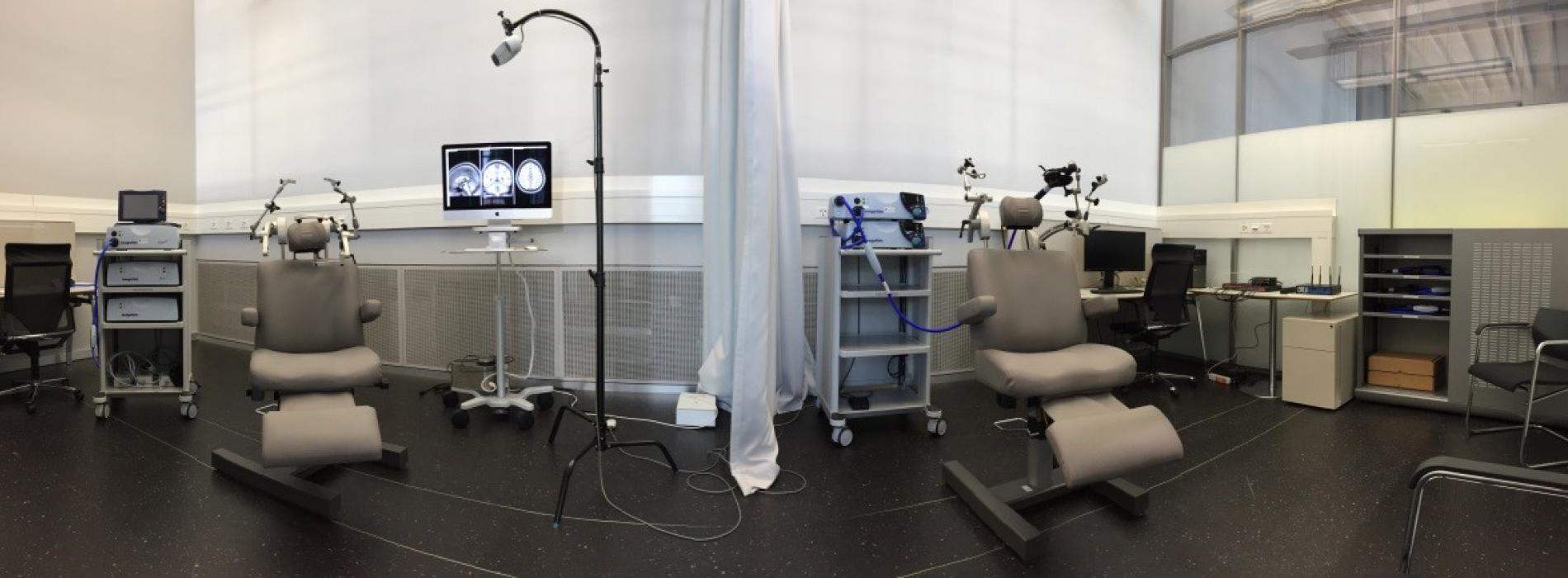
The mission of the Neuromodulation facility is to provide non-invasive magnetic (TMS) and electric (tDCS/tACS/tRNS, PNS, PAS) brain stimulation capabilities with the whole spectrum of applications, from virtual lesion approaches, over single and paired pulse mono-focal applications to determine intracortical interactions (e.g., gaba-ergic, gluatamate-ergic), bifocal applications to determine interregional interactions (e.g., interhemispherical) towards neuromodulation including modulation of oscillatory activity. This all can be done neuronavigated if excellent individual focality of the application is important.
In 2018, the neuromodulation platform welcomed the arrival of the world’s first MagVenture MagPro XP TMS stimulator dedicated to concurrent TMS-fMRI at 3T. It combines a high-power high-frequency repetitive TMS stimulator (up to 250 Hz TMS frequency) with an MRI-compatible TMS coil. Dedicated MRI coils allow to obtain the optimal MRI quality at the vicinity of the stimulation site, and record whole-brain high quality fMRI data at 3T. Find more information HERE.
The facility is dedicated to perform scientific projects using brain stimulation techniques in healthy subjects and also in patients with neuropsychiatric diseases. The close vicinity to the Clinical and Sleep Research Unit (CSRU) warrants adequate patient management. The CSRU is equipped to monitor clinically ambulatory patients, contains an automatized hospital bed, a tensiometer, a resuscitation trolley, a wheelchair and a nurse/TSM technician is present on site for medical assistance if needed.
Its vicinity to the other platforms, particularly EEG and MRI, is a considerable benefit and warrants to perform multi-modal research protocols in combination with non-invasive brain stimulation.
Scientific leadership of this facility is overseen by Pr. Friedhelm Hummel (EPFL) and Pr. Sophie Schwartz (UNIGE), while the neuromodulation facility is managed by Dr. Olivier Reynaud. Loan Mattera is the medical assistant.

Figure 1- Neuromodulation room at Campus Biotech
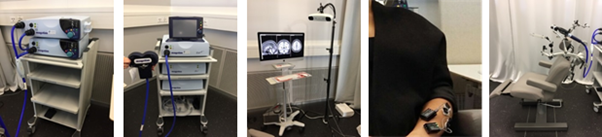
Figure 2. Neuromodulation equipment.
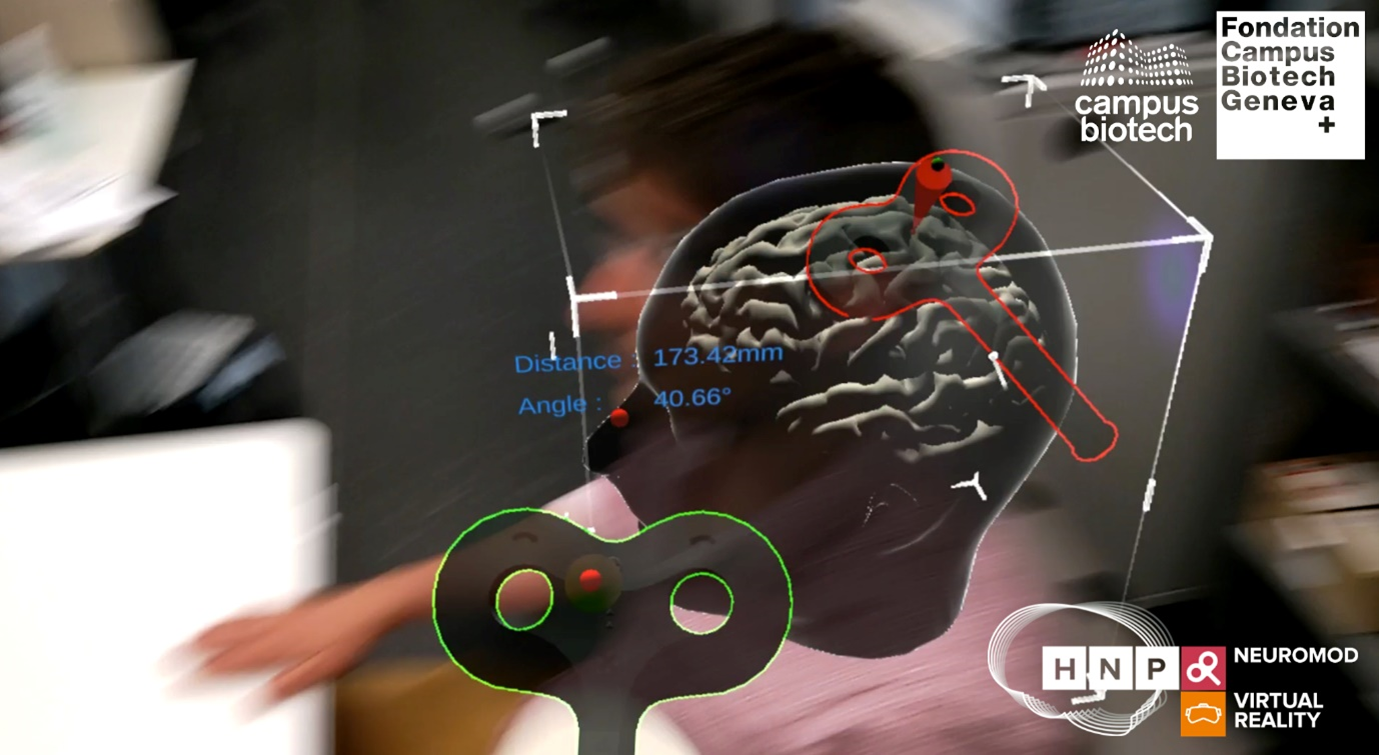
Figure 3. Non-invasive Brain Stimulation combined with Augmented Reality